Specific Heat Calculator
Use our specific heat calculator to calculate the energy required to achieve a desired temperature change, the mass, the change in temperature, or the specific heat capacity of a substance.
Heat Energy:
Heat Energy Formula
Heat Energy Formula
Heat Energy Formula
Heat Energy Formula
On this page:
How to Use Specific Heat Capacity to Calculate Heat Energy Transfer
Specific heat capacity, often simply called specific heat, is a property of a substance that describes how much heat energy it requires to raise the temperature of one kilogram of the substance by one degree Celsius (or one Kelvin). Different substances have different specific heat capacities, which means they require varying amounts of energy to achieve the same temperature change.
This property is crucial in many areas, from designing heating and cooling systems to understanding natural phenomena like ocean currents.
You can calculate the energy required to achieve a desired temperature change using the specific heat formula.
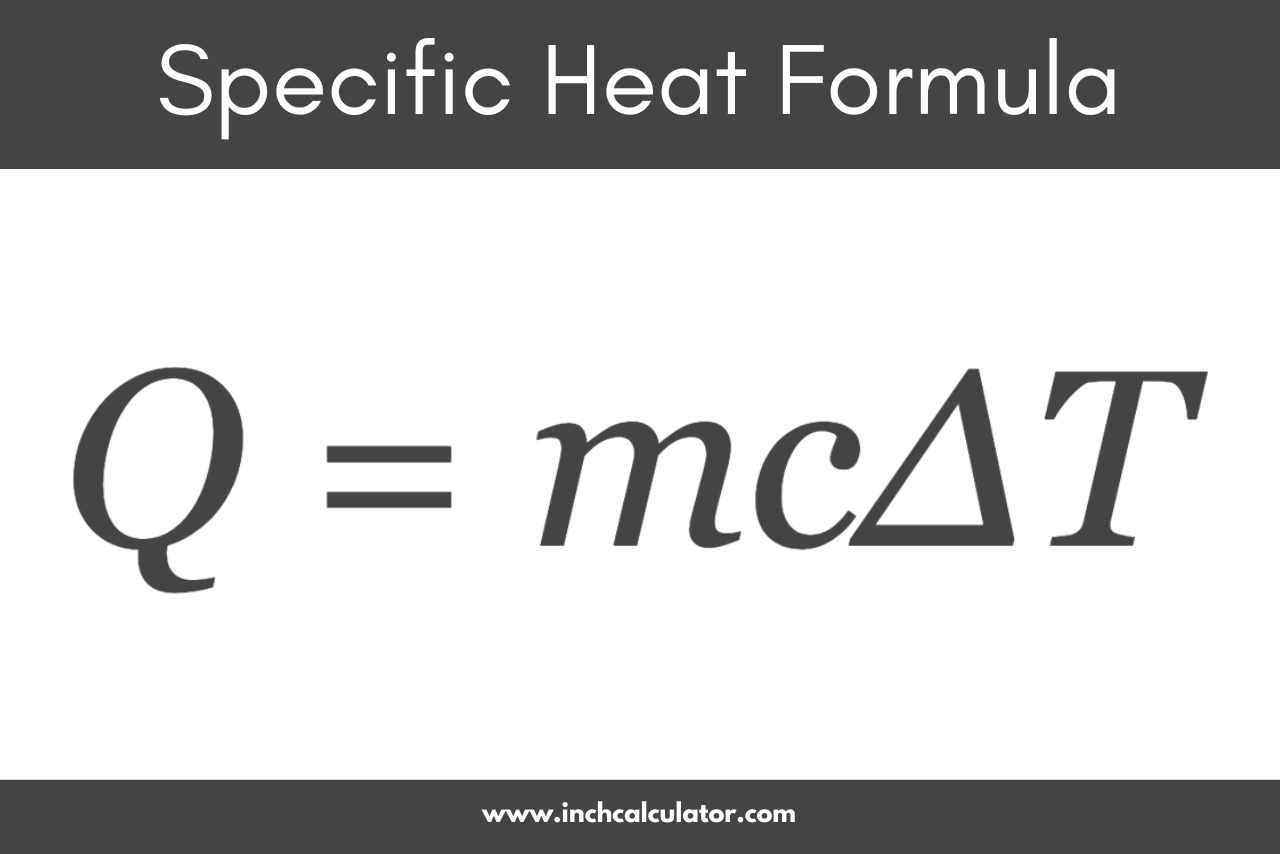
Specific Heat Formula
The specific heat formula is expressed as:[1]
Q = mcΔT
Where:
Q = the amount of heat energy in joules (J)
m = the mass of the substance in kilograms (kg)
c = the specific heat capacity of the substance in joules per kilogram per degree Celsius (J/kg°C)
ΔT = the change in temperature in degrees Celsius (°C) or Kelvin (K)
How to Use the Specific Heat Formula
Begin by identifying the mass of the substance, the initial and final temperatures (to determine the temperature change), and the specific heat capacity of the substance. These are the essential inputs for the formula.
Next, calculate the change in temperature by subtracting the initial temperature from the final temperature. Note that if temperatures are provided in Celsius, you don’t need to convert them to Kelvin as the degree size is the same in both scales.
Then, apply the formula by substituting the values of mass, specific heat capacity, and temperature change into the formula to calculate the heat energy in joules.
The final step is to interpret the results. The result will give you the amount of energy in joules required to achieve the specified temperature change in the given mass of the substance, which can help in understanding how different materials respond to heat and are used in various applications.
For example, let’s calculate the energy required to increase the temperature of 2 kg of water by 10 °C. The specific heat capacity of water is approximately 4,186 J/kg°C.
Q = 2 kg × 4,186 J/kg°C × 10 °C
Q = 83,720 J
Therefore, 83,720 joules of energy are required to heat 2 kg of water by 10 °C.
You might also be interested in learning how to calculate the energy needed to heat a home.
References
- Boundless, Physics - 13.2 Specific Heat, LibreTexts, https://phys.libretexts.org/Bookshelves/University_Physics/Physics_(Boundless)/13%3A_Heat_and_Heat_Transfer/13.2%3A_Specific_Heat

