Water Weight Calculator
Find how much water weighs given a volume in teaspoons, tablespoons, cups, quarts, pints, gallons, liters, or milliliters.
Results:
On this page:
- Water Weight Calculator
- How Much Does Water Weigh?
- How to Calculate the Weight of Water
- Weight of Water for Different Volumes
- The Temperature Affects the Weight of Water
- Density of Water at Various Temperatures
- How Much Does a Gallon of Water Weigh?
- How Much Do 5 Gallons of Water Weigh?
- How Much Does One Molecule of H2O Weigh?
- H2O Molar Mass
- References
How Much Does Water Weigh?
The weight of a volume of water can be found given the density, which is the mass per unit volume. The density of water is 1 kilogram per liter (kg/L) at 39.2° F or 4° C, but the precise density depends on the temperature.
1 kg/L is typically used to represent the density or weight of water at normal temperatures above freezing when very precise measurements are not required.

Therefore, 1 liter (L) of water weighs 1 kilogram (kg) and 1 milliliter (mL) of water weighs 1 gram (g).
In common US measures, one gallon of water weighs 8.345 pounds.
The density of water varies slightly at different temperatures, which affects the precise weight per volume.
How to Calculate the Weight of Water
To find the weight of water, start by using the standard density (1 kg/L at 39.2°) and the volume of water in liters. Multiply the volume of water in liters by the density to find the weight.
You can use our volume converter to convert different volumes to liters.
For example: let’s find the weight of 250 mL of water.
Step one: convert volume to liters:
Volume (L) = 250 mL ÷ 1000 = 0.25 L
Step two: using the density of 1 kg/L, calculate the weight of 0.25 L of water:
Weight (g) = 0.25 L × 1 kg/L = 0.25 kg = 250 g
Thus, 250 mL of water weighs 0.25 kilograms or 250 grams.
1 gram of water is equal to 0.035274 ounces, so to get a result in ounces, simply multiply the grams by 0.035274. You can also use our weight converter to convert from grams and kilograms to pounds and ounces.
Weight of Water for Different Volumes
| Volume | Weight (oz) | Weight (lb) | Weight (g) | Weight (kg) |
|---|---|---|---|---|
| 1 teaspoon | 0.1739 oz | 0.0109 lb | 4.929 g | 0.004929 kg |
| 1 tablespoon | 0.5216 oz | 0.0326 lb | 14.787 g | 0.0148 kg |
| 1 cup | 8.345 oz | 0.5216 lb | 236.59 g | 0.2366 kg |
| 1 pint | 16.691 oz | 1.043 lb | 473.18 g | 0.4732 kg |
| 1 quart | 33.382 oz | 2.086 lb | 946.35 g | 0.9464 kg |
| 1 gallon | 133.53 oz | 8.345 lb | 3,785.4 g | 3.785 kg |
| 1 milliliter | 0.0353 oz | 0.002205 lb | 1 g | 0.001 kg |
| 1 liter | 35.274 oz | 2.205 lb | 1,000 g | 1 kg |
| 1 cubic inch | 0.578 oz | 0.0361 lb | 16.387 g | 0.0164 kg |
| 1 cubic foot | 998.85 oz | 62.428 lb | 28,317 g | 28.317 kg |
| 1 cubic yard | 26,969 oz | 1,685.6 lb | 764,555 g | 764.55 kg |
| 1 cubic centimeter | 0.0353 oz | 0.002205 lb | 1 g | 0.001 kg |
| 1 cubic meter | 35,274 oz | 2,204.6 lb | 1,000,000 g | 1,000 kg |
The Temperature Affects the Weight of Water
As we mentioned above, temperature affects the density of water, and thus its precise weight will vary with the temperature. As the temperature of water rises, it expands, causing it to increase in volume slightly.[1]
At a molecular level, as the temperature rises, the heat of the water molecules increases, which increases their energy. As the energy in the molecules rises, the particles within move and vibrate more, causing them to take up more space.
Thus, the warmer the water is, the more volume it will consume and the lower its density will be.
Density of Water at Various Temperatures
The chart below shows the density of water at various temperatures, according to the US Department of the Interior.[2]
| Temperature ( °F / °C ) |
Density ( g/cm3 ) |
|---|---|
| 32° / 0° | 0.99987 g/cm3 |
| 39.2°/4.0° | 1.00000 g/cm3 |
| 40°/4.4° | 0.99999 g/cm3 |
| 50°/10° | 0.99975 g/cm3 |
| 60°/15.6° | 0.99907 g/cm3 |
| 70°/21° | 0.99802 g/cm3 |
| 80°/26.7° | 0.99669 g/cm3 |
| 90°/32.2° | 0.99510 g/cm3 |
| 100°/37.8° | 0.99318 g/cm3 |
| 120°/48.9° | 0.98870 g/cm3 |
| 140°/60° | 0.98338 g/cm3 |
| 160°/71.1° | 0.97729 g/cm3 |
| 180°/82.2° | 0.97056 g/cm3 |
| 200°/93.3° | 0.96333 g/cm3 |
| 212°/100° | 0.95865 g/cm3 |
How Much Does a Gallon of Water Weigh?
We mentioned above that one gallon of water weighs 8.345 pounds at 39.2 °F when the density of water is exactly equal to 1.0 g/cm³. But, since the density of water fluctuates with the temperature, the weight of a gallon of water also changes slightly.
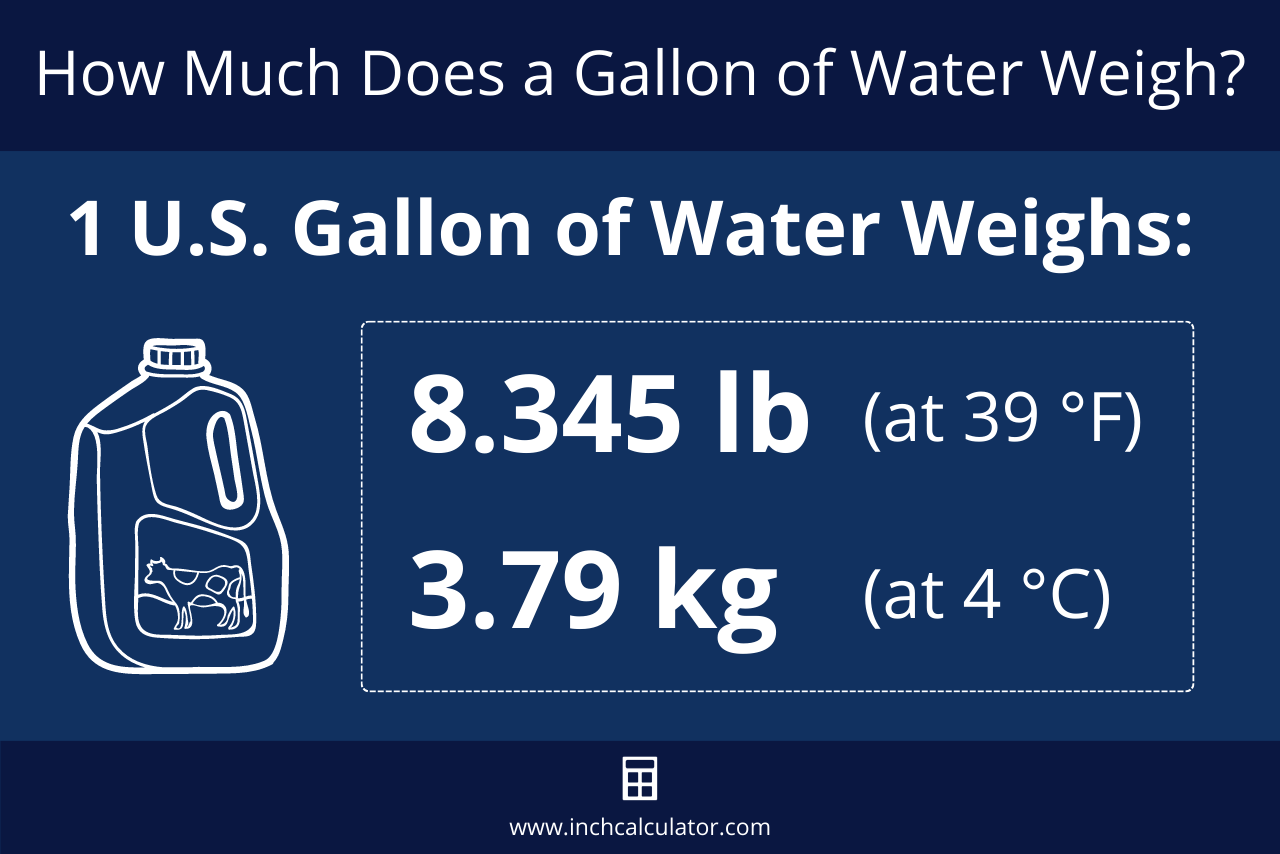
So, just how much does a gallon of water weigh? One gallon of water weighs between 8.288489 and 8.345404 pounds (lb), depending on the temperature. The table below shows the weight at various temperatures.
| Temperature ( °F / °C ) |
Weight of One Gallon of Water | Weight of Five Gallons of Water |
|---|---|---|
| 32° / 0° | 8.34432 lb | 41.721598 lb |
| 39.2°/4.0° | 8.345404 lb | 41.72702 lb |
| 40°/4.4° | 8.345321 lb | 41.726605 lb |
| 50°/10° | 8.343318 lb | 41.71659 lb |
| 60°/15.6° | 8.337643 lb | 41.688215 lb |
| 70°/21° | 8.328881 lb | 41.64405 lb |
| 80°/26.7° | 8.317781 lb | 41.588905 lb |
| 90°/32.2° | 8.304512 lbs | 41.52256 lbs |
| 100°/37.8° | 8.288489 lb | 41.442445 lb |
How Much Do 5 Gallons of Water Weigh?
Trying to figure out how much a 5-gallon bucket of water weighs? At room temperature, five gallons of water weighs 41.64 pounds, but at 39.2 °F, it weighs 41.727 pounds.
You can use the calculator or weight chart above for the full five-gallon weight range.
Working on a plumbing project? Use our pipe volume calculator to calculate the volume and weight of the water in your plumbing system.
How Much Does One Molecule of H2O Weigh?
In chemistry, water is also referred to as H2O. It’s an oxygen hydride that consists of a single oxygen atom bonded to two hydrogen atoms.[3]
To determine the weight of an H2O molecule, you need to first find its molar mass.
H2O Molar Mass
You can find the molar mass of H2O by finding the atomic mass of each element in the molecule and adding them together.
The mass of hydrogen is 1.00794 g/mol, and oxygen is 15.9994 g/mol. Since water contains two hydrogen atoms and one oxygen atom, the molar mass formula for H2O is:
H2O molar mass = 1.00794 + 1.00794 + 15.9994
H2O molar mass = 18.01528
Therefore, the molar mass of H2O is 18.01528 g/mol, or 18.01528 grams per mole.
After finding the molar mass, you can find the weight of a molecule using Avogadro’s constant, which states that one mole is equal to 6.02214076 × 1023 elementary units of matter, such as a molecule. That’s a pretty large number, so it’s typically represented using scientific notation.
Using this constant and the molar mass above, the formula to find the weight of one H2O molecule is:
H2O (g/molecule) = 18.01528 (g/mol)/6.02214076 × 1023 (molecule/mol)
H2O (g/molecule) = 2.9915 × 10-23 g
So, one molecule of H2O weighs 2.9915 × 10-23 grams.
References
- U.S. Department of the Interior, Bureau of Reclaimation, Ground Water Manual, from The Water Encyclopedia, Third Edition, Hydrologic Data and Internet Resources, 1977, Edited by Pedro Fierro, Jr.
and Evan K. Nyler, 2007, https://www.usbr.gov/tsc/techreferences/mands/mands-pdfs/GndWater.pdf - U.S. Department of the Interior, US Geological Survey, Water Density, https://www.usgs.gov/special-topics/water-science-school/science/water-density
- National Center for Biotechnology Information, PubChem Compound Summary for CID 962, Water, 2021, https://pubchem.ncbi.nlm.nih.gov/compound/Water


